A Brief History of the SARS-CoV-2 Coronavirus Pandemic
We live in evolutionary competition with microbes—bacteria and viruses. There is no guarantee that we will be the survivors.
Joshua Lederberg
Teacher, Geneticist, Nobel Prize Winner, Good Guy
As an undergraduate I studied a virus called T4. That lab was my introduction to the power of viruses and molecular biology and working there remains one of my happiest memories. The place was a refuge where I could do experiments that had never been done before; it had interesting people and good scientists from all over the world, as did the neighboring labs. My boss and mentor, Chris Matthews, was 28, older than I was but not by much. He was already an Assistant Professor. That was 1966, but all these years later Chris and I have avoided senescence or at least its worst effects. He reminded me recently that we published a good paper in the inaugural issue of The Journal of General Virology.
T4 virus has a surreal form: under the lens of an electron microscope, it looks like the first moon-lander, and it has a similar job: land at a particular protein on the surface of a bacterial cell. This tiny machine has spindly legs and a spike that is essentially a syringe. Above the spike, where the lander’s capsule would be, a hollow body contains the virus’s DNA cargo. The tube contracts to blast that DNA across the cell wall and surface membrane and into a bacterial cell. The DNA contains information that automatically makes more copies of the virus and then destroys the bacterium.
Despite its austere name, T4 virus has a certain cachet in the world of molecular biologists because of all the information scientists have derived from it. What is the genetic material? How does it mutate? What are the physical properties of a gene? How do genes exchange segments? How do simple nanomachines like T4 turn on their genes in a particular order? How do nanomachines like T4 assemble the copied DNA into the capsule and then attach the tail? T4 and similar viruses still provide tools to study fundamental problems in biology.
The wonderful thing about T4 for an undergraduate was that it works fast: experiments lasted a day, or more likely a night. When I added a few T4 viruses (or bacteriophage, as they are called) to a growing culture of E. coli and incubated the test tube at human body temperature (37 deg C), nothing seemed to happen for half an hour and then poof, the cloudy solution disappeared, leaving a slightly opalescent clear liquid. One or two viruses per bacterial cell had become 100; and in an elegantly timed last act, the virus made an enzyme that dissolved the bacterial cell from within, liberating the new viruses. I was left with one milliliter in a glass tube. Chris noticed my astonishment and grinned. “Impressive, isn’t it? How many viruses are in there, do you think?” About 10 billion, as it turned out.
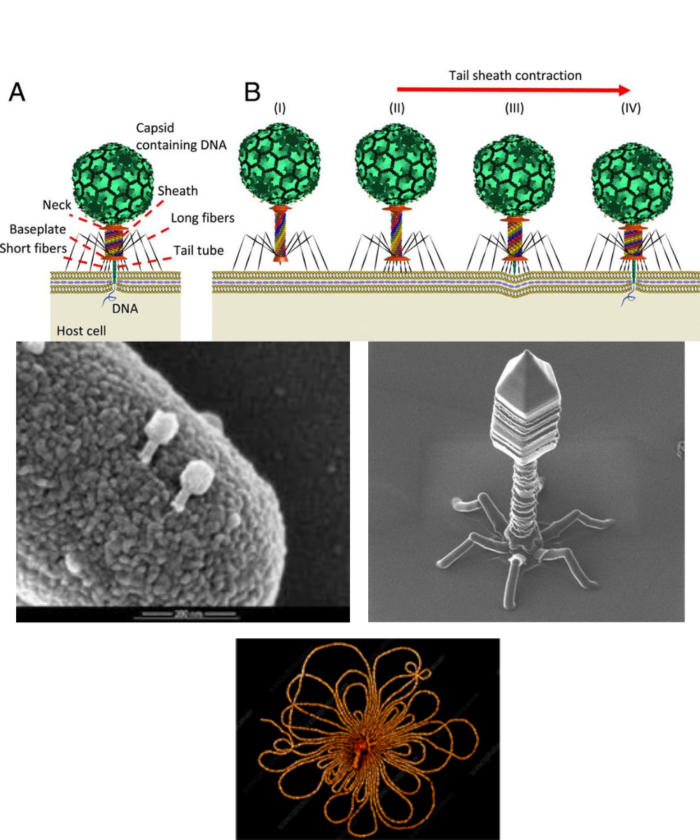
Bacteriophages were discovered by Felix d’Herelle in the 1920s. He and others thought they might have therapeutic potential, but despite some success, the field did not develop. Interest has revived. In the upper panel, the virus has landed on a bacterium; the stem of the bacteriophage contracts like a syringe and forces the DNA into the bacterial cell. See: Ameneh Maghsoodi et al, https://doi.org/10.1073/pnas.1909298116. In the next panels viruses have been coated with a metal spray and a scanning electron microscope has outlined its extraordinary shape. The last panel shows DNA, released from the still visible phage particle by osmotic shock.
All viruses are intracellular parasites and have the same modus operandi: bind to a specific protein on a cell surface, get their DNA, (or sometimes their RNA), inside, make more viruses at the expense of the host cell, and get out. There are variations but a virus has no intent or malevolence; infection is mechanical. We like human metaphors in our explanations of nature, but the virus is just business.
The events get more complicated when a virus confronts immune systems or other defenses, as we will see with SARS-CoV-2. No invader, even a virus, attacks a cell without having the means to combat the host’s defenses, which are elaborate and include an adaptive antibody producing system that takes time and an innate immune system that is always active. But in 1966, I was not concerned with the defense systems of bacteria or the immune systems of mammals. Much less was known about immunology then and we took pride in simple focused experiments. Bacteriophages were the thing to work on. That has changed and immunology is now king, even for diehard bacteriophage partisans.
Viruses are small and because they pass through a fine filter that retains bacteria, they were called filterable agents. Attempts to target viruses with drugs usually fail because they use the machinery of our own cells. Antibiotics, in 1965 the glory of medicine, do not work against viruses. Heads of infectious disease societies declared antibiotics had defeated infectious disease, or to use one author’s phrase: turned the page on infectious disease. Hardly. Still, by the 1950’s and 1960’s science and medicine had bested polio, measles, rabies, mumps, rubella, chickenpox, whooping cough, diphtheria, and other virus and bacterial diseases with vaccines and antibiotics. Smallpox was eliminated in the late 1970’s. Tuberculosis could be treated from the early 1950’s. Since about 1995, new classes of drugs have worked spectacularly against HIV and Hepatitis C viruses, but these were unimagined in 1965, as were DNA sequencing, molecular cloning, monoclonal antibodies, and other tools that are now routine.
Since the Body Scientific column began in The Lakeville Journal in 2010, there have been several viral defined as vast infectious events covering continents. H1N1 influenza in appeared in Mexico in 2009, H5N1 and several other flu viruses showed up after that. We rediscovered Ebola, Zika, and Chikungunya viruses in 2014 and 2015. The world is in its 7th cholera pandemic, although the disease is rare in the United States, reminding us that public health measures are always the best defense against disease. Virologists, chastened by the 1918-1919 flu epidemic, thought another flu virus would drive the next big pandemic, but were slammed by SARS-CoV-2. Scientists and physicians were aware of coronaviruses because in 2003 the world had an epidemic of SARS, which infected 8000 people, killed 800, and then disappeared. Coronaviruses also infect camels (MERS or Middle Eastern Respiratory Syndrome) and the people who handle them, for whom it is exceptionally lethal, reaching 40%. Other coronaviruses cause mild colds and may provide some protection against to SARS-CoV-2, if antibodies that cross react with SARS-CoV-2 are produced during these colds. What links different SARS viruses is the similarity of their and they look alike under an electron microscope.
Scientists and physicians tend to be optimists and proud of their abilities, but that attitude can give the impression all problems can be easily solved. With a new disease or even an old one, this confidence is never a given. We have failed, so far, to make good vaccines for HIV, trypanosomes, malaria, amoeboid diseases, or Hantavirus, among others, including those lurking in host animals, waiting to infect humans. Skepticism is a scientific virtue and so is humility. The Body Scientific has been preaching their value since 2010. We recommend genial skepticism, though. Yelling at people does no good, although I confess to the occasional failure.
By historical standards the SARS-CoV-2 vaccines whose use is spreading across the United States and other countries are a triumph, but I agree with a statement by President Biden’s press secretary, Jen Psaki. When asked about who deserves credit for the vaccines and their distribution, she replied that with more than 600,000 dead Americans and millions of victims throughout the world, no one should claim much credit.
This story of Covid-19 began with columns I wrote from February 2020 to February 2021. In this narrative I have modified the columns because with new information coming at fire hose volume, leaving them intact was not useful to readers. I have retained some of the order, which imposes a sense of timing and allows us to ask which of my assumptions, or those of other people, proved wrong. Errors and misconceptions are inevitable and essential in dissecting scientific problems. The same thinking applies to after-action reports in the military, case studies in medicine, and National Transportation Safety Board accident investigations. The mistakes I made in the columns I wrote since January 2020 are proudly noted in brackets. Progress since the original columns were written appear in parentheses.
A Strange Pneumonia in Wuhan
Of the scourges the natural world can throw at us, a lethal new virus is one of the most frightening. At first, we don’t know where the virus came from, how many victims it will kill or leave debilitated, how to treat it, or how far and fast it will spread. Viral pandemics seem apocalyptic, and the response is often fear, or worse, panic. In March 2020, we were in the fear stage for Wuhan coronavirus (as it was then called), but science and industry will chip away at its biology, while it also deploys to make vaccines and other treatments. That has certainly happened. Technology and years of hard work have given us new weapons, but public health measures are often the most important tools at the beginning of pandemics.
Openness is the first rule of public health, even if the news is bad, otherwise no one has confidence in the public health authorities. The (severe acute respiratory syndrome (SARS) infection of 2003 was kept secret for months by Chinese government authorities, giving the virus time to spread within China and abroad. Eventually, quarantine and public health measures suppressed it, but not before 800 people died of about 8000 diagnosed patients. The damage to trust between governments and scientists was severe and remains so. This reticence of authoritarian governments to admit bad news has happened again with SARS-CoV-2.
Dr. Li Wenliang first noticed the “pneumonia of unknown origin” cases in 2019, as part of an informal surveillance system that he and other physicians had organized. When he reported these observations to his hospital colleagues in Wuhan, he was criticized by the police for spreading rumors and forced to sign a confession that he was spreading rumors. Such are the dangers of science in authoritarian societies.
Once the problem expanded and was undeniable, Chinese scientists and physicians were aggressive in efforts to study, treat, and contain the virus, but as I learned from Bob Woodward’s book, Rage, CDC investigators were kept out despite repeated requests by Dr. Robert Redfield, the Director of our CDC to his counterpart in China, Dr. George Fu Gao. According to Lawrence Wright in the January 4, 2021, issue of the New Yorker (and now in a book), Dr. Gao was upset (Wright’s story says in tears), that he could not invite the colleague whom he had known for years. By this time, Chinese scientists were learning about a new and contagious virus was on the loose. In February 2021, a World Health Organization delegation made an inconclusive visit. The visit stirred more controversy, turning into an international issue. We still do not know the original reservoir of this virus.
Chinese scientists and physicians are probably not to blame for the breach in trust, but rather security officials and other bureaucrats marinating in the suspicions of an authoritarian regime. Dr. Li Wenliang, an ophthalmologist, died in February 2020 of Covid-19 contracted while he was performing eye surgery on an elderly woman. His obituary appears in the URL below his photograph. If courageous and informed people are silenced, it does not bode well for China or any other country.

Dr. Li Wenliang first noticed the new respiratory syndrome and reported it to his colleagues. Dr. Li died in February 2020 of the disease he had discovered.
Read his obituary at https://doi.org/10.1016/ S0140-6736(20)30382-h2

Dr. Shi Zhengli of the Wuhan Institute of Virology and her colleagues determined the sequences of the viral RNA genome from four patients with Covid-19. On January 10, she sent the sequence to Genbank, an NIH repository for virus and other genetic sequences. That led to the Moderna, Pfizer, and other vaccines.
The treatments of the first 99 patients in Wuhan were described in the January 29, 2020, issue of The Lancet, the British medical journal. Half of the patients worked in a live animal food market that was an immediate source of the epidemic, but not the original source of the novel coronavirus. According to Chinese authorities, by February, the virus had spread to all Chinese provinces. As of Feb. 4, 2020, there were 24,391 confirmed cases and 479 deaths, almost all in China. There were 1015 confirmed recoveries. There was person-to-person transmission, through the air, which was frightening because it is very hard to contain. 2019nCoV (now SARS-CoV-2) is not as communicable as measles, nor as lethal as Ebola, MERS, or SARS. It turned out to be bad enough.
Of the eleven Wuhan patients who were initially admitted to hospital, a number were smokers or with compromised health. The Lancet paper described extensive efforts to help these patients, including ventilation and the use of experimental drugs. About half died. The remaining patients either recovered or were no longer critical at the end of January.

Transmission electron microscopic image of an isolate from the first U.S. case of COVID-19. The spherical viral particles contain cross-sections through the viral genome, seen as black dots. This is a thin slice through an infected cell. From the NIH image library.

A free coronavirus is about 80 billionths of a meter in diameter. It is hard to imagine something so small, let alone how dangerous it is. A thousand lined up would be about the width of a hair. From the CDC image library: https://www.cdc.gov/media/subtopic/images.htm.
The United States had 11 known cases of Covid-19 as of Feb. 4, 2020—all people who had arrived recently from Wuhan and were isolated until they recovered. Local health authorities traced and talked to all contacts, providing food, thermometers, and other necessities. Whether those 11 cases would give rise to new ones was the question at the time. [We know the answer was no. Frankly I was hopeful, but the CDC was not. They were right].
There is a lot to learn during a new infection; an infection is not a sudden event like a gunshot wound. It is a gradual process, especially in the airways. Entering respiratory virus may be pushed up by cilia, millions of little waving oars that drive mucus and fluid upward in the lungs and trachea. Clearing the throat and swallowing kills viruses which dissolve in stomach acid. Virus entry may be blocked by a coating of mucous on the cells of the bronchi or trachea; airways are lined with cells that secrete mucus. Sometimes the virus gets through these physical defenses and at that point, other tools of the immune system respond, as we will see. The host has many defenses against invaders, but the invading pathogen also brings weapons to stifle the defenses of the host.
My late colleague, Anthony Piel, former counsel to the World Health Organization, pointed out on February 6, 2020, that pandemics can be stifled if nations cooperate. Otherwise, there are too many types of viruses, and they evolve very fast. The United States has always been a leader in Public Health and disease control. We had a research program called Predict that searched for novel viruses before they jumped to humans, but that program was stopped, at least temporarily. A pandemic response plan was shelved. A link to it can be found at the end of this essay. The United States quit the World Health Organization in an act of petulance and blocked a long-standing cooperative effort to let an American lab collaborate with the Wuhan Institute of Virology with NIH support. We knew what viruses they had sequenced. Anger is dangerous in a pandemic. Nobody thinks clearly when they are angry.
Bats, Viruses, and Evolution
Caves shelter billions of bats, from Hunan in China to Carlsbad in New Mexico. Bats are the only flying mammals; they fly out at night to feed on insects, fruit, or for vampire bats, blood. Their most extraordinary ability is echolocation; they listen in the dark and navigate by sonar, which allows them to find food, evade predators, and get back to their caves. They fly long distances, leave droppings widely and some of them bite. They are unique vectors of disease. We think of rabies as a disease of the past, but in the Amazon forests thousands of people die of rabies from bat bites every year. Many viral pathogens have been found in bats: Nypah virus, rabies virus, and coronaviruses, including SARS1 and SAR-CoV-2, to name some. These viruses are often zoonotic, which means they have jumped from animals to humans through a bite, food contamination, or because people eat bats or other infected animals.
In the winter, bats hang from the roof of caves while they hibernate and even on temperate days, they spend time there. Their droppings accumulate as an ammonia-rich slush on the cave floor, which sounds revolting, but all that nutrient-rich guano harbors and feeds an extraordinary community of organisms, including coronaviruses and strange predatory amoebae (a tale for another time). People harvesting this guano for fertilizer are sometimes infected. Dr. Shi Zhengli and her colleagues at the Wuhan Institute of Virology sample these environments, bring the specimens back to their level 4 biosafety lab and determine the sequence of any viruses they can grow in cultures of human cells. It can be dangerous work. Dr. Shi’s laboratory is presently at the center of a conflict about how SARS-CoV-2 got loose on the world. Did they make the virus? Did SARS-C0V-2 virus escape the lab? Or was it a natural isolate that was ferried out of a cave by a guano harvester or scientist? I lean toward some version of the last, but there is no way to be sure and Chinese Communist Party paranoia is not helping.
……..
The March 12, 2020, issue of Nature, an international scientific journal, has two dense papers on the discovery and sequence of SARS-CoV-2. Though most of the data were already available, the narrative, assembled in one place, is gripping. The first article describes a 41-year-old man, straining to breathe, who was seen in The Central Hospital of Wuhan on Dec. 26, 2019. Four other cases in the second paper were confirmatory; the patients were all infected by the same virus. The first victim had been sick for six days and reported fever, chest tightness, unproductive cough, pain, and weakness. Influenza, which might have explained the symptoms, was ruled out. He needed help breathing and had abnormal lungs on chest X rays and CAT scans.
Twelve days after the appearance of symptoms, the patient landed in intensive care. His physicians put saline deep into one lung with a thin catheter and recovered 200 microliters of saline wash fluid— about four drops. They hoped to flush out a virus, and they did—about 100 million particles. Virologists and biologists are used to large numbers, but 100 million in 4 drops (0.2 milliliters) is a lot of viruses. Images of viruses leaving human cells are consistent, as in Figure 4. SARS-CoV-2 infections keep on producing until the host cell perishes by T-cell attack or apoptotic cell death, a well-studied form of cellular suicide that limits virus spread in mammalian or vertebrate hosts.
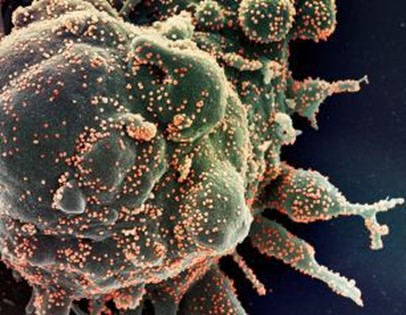
This ugly cell has produced many thousands of SARS-CoV-2 viruses, shown as orange specks. Each speck contains a virus on the way out of the cell. The cell is probably deformed because it is undergoing apoptosis. This scanning electron microscope image of a cell has been magnified thousands of times. The coloring has been added by computational techniques. Courtesy of the NIH.
Virus genomes are made of four nucleotide subunits: A, C, G, and U. In a particular order they provide coded instructions for a cell to make viral proteins, like an alphabet provides components for words. Think of the genome shown here as a long sentence with semi-colons. When virologists at The Wuhan Institute of Virology and another group in Shanghai sequenced the virus, they found it contained almost 30,000 nucleotides. They learned they had a new and possibly dangerous coronavirus that was related to the SARS virus of 2003, to MERS, the virus of camels, and coronaviruses that cause colds. In extensive computer comparisons—a kind of 23 and Me for viruses—they learned the new virus was also related to a bat coronavirus that they had found previously on expeditions to caves in Yunnan, but the new virus was not identical to that or any other known virus. It still differed by about 1500 changes from the nearest relative SARS virus.
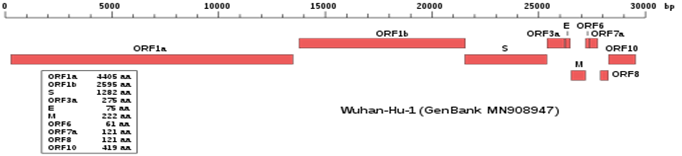
The SARS-CoV-2 genome contains information to make three large proteins: ORF1a and ORF1b provide information to make an RNA polymerase and a protease, respectively. S provides instructions for the famous Spike Protein. ORF stands for Open Reading frame, which means that the sequence has no interrupting mutations and has the information to make a protein. The box shows the length of the proteins in numbers of amino acids. Some of the sequences at the end of the virus genome code for small proteins that inhibit a lung cell’s immune system. See the colored map below. This is the gene structure of the original virus isolated and named in Wuhan: Wuhan-HU-1. The number GenBank assigned it is: MN908947.
In Wuhan scientists were concerned about the origins of the virus. They thought that the virus had made its way into a local indoor fish and seafood market where their patient and other infected people had worked. The market sold live hedgehogs, badgers, snakes, and doves, and the new coronavirus may have infected one of these species before it jumped to humans. The reservoir species is still unclear. Pangolins, perhaps. Information is not being released.
By the middle of January 2020, the novel pneumonia had spread, affecting more that 100,000 people in Wuhan and had killed thousands. Hospitals were overwhelmed, despite the efforts of the authorities to build new hospitals and an enforced stay-at-home policy to break the chain of infection. At first there were not enough supplies, nurses, or physicians to treat all patients. A lockdown slowed the contagion. This is the situation the United States, Italy and other countries would soon be in. (It was exactly the situation we were in. At the time, many of us thought that in most places in the United States, such a stringent lockdown would be impossible. We were mostly right).
Epidemics and the speed at which a disease spreads have been studied for a long time and mathematics has made its contribution with a factor called R0 (pronounced R naught). It describes the number of people a single patient infects, on average. With measles5, R0 is very high, 12 to 18—think of the measles infection in Disneyland a few years ago. SARS-Cov-2 is less infectious than the measles virus, and its R0 is estimated to be 2-3. (The mutations from the UK and South Africa variants add about 0.4 to that number, according to one report I read. One R0 estimate for the delta variant with nearly 8. R0 for most influenza viruses is about 1.3. An R0 value above 1 means that an epidemic will expand; reducing R0 to 1 lets the infection smolder in the population. Reducing it to less than 1 drives the virus to extinction, as happened with the first SARS virus in 2003. Reducing R0 is the idea behind sheltering in place, hand washing, and personal protective gear, including masks.
Most of the viruses that afflict us use RNA, not DNA, for their genomes. Other than in viruses, this is rare. I do not know why viruses use RNA, except that once the SARS-CoV-2 genome is in the cell, it can make new proteins and virus immediately. The dogma of molecular biology has been that DNA makes RNA; RNA makes proteins, and proteins can do almost anything, including making structural elements and providing thousands of chemical catalysts called enzymes. Since the chemical structure of RNA differs a little from DNA, the virus brings enzymes that our cells do not have. One enzyme tacks the subunits of RNA together to make a genome copy (an RNA-dependent RNA-polymerase). Another enzyme is a protease that cuts proteins into smaller functional units, as many viruses do. Inhibitors that block the action of these enzymes stop a virus from reproducing, but do not affect human cells. Inhibitors of similar enzymes in HIV and hepatitis C virus have essentially controlled these diseases. A recent report in the New England Journal of Medicine tested two inhibitors that block HIV but failed to stop SARS-Cov-2. Remdesivir has been approved, but questions remain about how well it really works on SARS-C0V-2. At the end of January 2021, two new drugs, tocilizumab and sarilumab that suppress inflammation have proved useful. Others will appear [they have]; it took 10 years and a lot of excellent chemistry to produce the drugs that now control HIV and hepatitis virus.
In March 2020, reports from China and France suggested that a combination of hydroxychloroquine, an antimalarial drug and azithromycin, an antibiotic, stopped coronavirus reproduction. These reports described tests on small numbers of patients and on closer examination the experiments proved to be a muddle. These drugs should not have been promoted as a treatment for the infection. Their lead author has been called before French scientific authorities to answer questions about the reliability of his work. (In larger clinical trials the drugs have turned out to be useless, either to control the infection or to block it from starting.)
Fear, Action,
and a fastball, low and away.
What the natural world can mount in the way of threats is greater than any disease organism we could build ourselves. The universe of viruses has ‘world enough and time’ to create new viral genomes by mutation or exchange between different viruses. All genomes mutate. Nature has the advantage of vast numbers of diverse genomes, time measured in years, and selective pressure—if these viruses do not change, they will be wiped out by immune systems in the animals they infect. When a new disease appears, we tend to blame humans, as if nature could not be so destructive without human help. Nature is not clever, but it does not need our help. Alas, making a virus worse is not beyond human capacity.
The genome of SARS-CoV-2 is almost 30,000 nucleotides long. It had taken the scientists at the Wuhan Institute of Virology and universities in Shanghai ten days to sequence and analyze the genome. The instructions for making SARS-CoV-2 were described in Nature in early March 2020, but the sequences had arrived at the NIH on January 10. That the Wuhan Institute of Virology director deposited the sequence so fast is not the act of scientists hiding information. Perhaps the functionaries of the Chinese Government knew nothing about sequencing or GenBank. My guess is the critical sequence information whizzed by them, like a fastball, low and away.
From that sequence, skilled virologists can make vaccines and begin other studies. Dr. Barney S. Graham is an expert on how to make a virus provoke the human immune system. He had been working on RNA viruses and had created a vaccine against Respiratory Syncytial Virus, which causes a serious and common pulmonary disease in children. His department quickly switched to Covid-19. One of the research fellows at NIAID, Dr. Kizzmekia Corbett, did much of the direct analysis and work. Having done the basic work on SARS-CoV-2, at the National Institute of Allergy and Infectious Diseases, NIAID licensed the project to Moderna, a company with expertise in RNA vaccines.
Between April 20, 2020, when this column first appeared and the widespread availability of vaccines, the best we could do, beyond testing and isolation, was to find drugs that slow the virus infection and protect front-line nursing and medical staff. We should wear masks ourselves (see below). There will probably be a new surge of virus in the fall (there has been) and it would be criminal to ask nurses, doctors and critical medical staff to return to emergency rooms and intensive care units without much better protection than they have had.

Barney Graham, MD, PhD and Kizzmekia S. Corbett, PhD work at the National Institute for Allergy and Infectious Diseases. They have worked on vaccine and monoclonal antibody development for SARSCoV-2, RSV, influenza, Nipah, Zika, HIV, Ebola, and other emerging pathogens. Dr. Corbett is a research fellow and designed the genetic element used to produce the Moderna vaccine. Dr. Corbett will continue her vaccine research as an Assistant Professor at the Harvard T.H. Chan School of Public Health.
Several vaccine candidates entered phase 1 clinical trials in the late spring and early summer of 2020. They are tested for safety, dose, and the immune responses they provoke. The vaccines usually present humans with the coronavirus Spike protein whose role is to bind to a protein that protrudes from the surface membrane of human cells. Starting in February 2020, it will take at least a year to complete Phases 2 and 3. There will be intense pressure to speed the process up. (The timing turned out to be right and we are perhaps doing better than expected.
Drugs that slow viruses like SARS-CoV-2 are also entering clinical trials. Remdesiver blocks the production of the RNA genomes for new viruses. More trials are necessary according to Dr. Anthony Fauci, for whom this is familiar territory from the battle against HIV. Tests of dosage and early use in the course of an infection may make remdesiver more effective, but there were mixed results at the beginning. Another drug, baricitinib, reduces the cytokine storms and inflammation that occur days after infection. The NIH established a clinical trial that asks whether the two drugs have additive benefits. A drug or vaccine does not have to be completely effective; it needs to tip the balance in the patient’s favor and remdesiver seems to do that.
Prof. Arturo Casadevall of The Johns Hopkins Bloomberg School of Public Health speaks of layered defenses. What he has in mind is the convalescent antisera of people who have recovered from Covid-19. This approach to stopping the virus by supplying antibodies to the circulation has entered clinical trials in the United Kingdom and will soon be in double-blind trials in the United States (it is). Anecdotal evidence (a scientific oxymoron) suggests that Italian patients benefitted from convalescent serum. What seems to be happening is that post-immune serum and particularly combinations of monoclonal antibodies mitigate the disease, but only if given early in the infection (January 2021). SARS-CoV-2 virus makes a huge number of copies of itself, and it may be essential to intervene when there are fewer viruses, or less damage has been done from inflammation.
Spread of SARS-CoV-2 in the United States
In February and March 2020, there was increasing community spread of SARS-CoV-2 virus in the United States. Community spread means that the infection cannot be traced to a single source and that the virus is loose in the population. The first American fatalities came from a nursing home in Washington State, but soon the epidemic no longer has a single focal source. In March 2020 growth seemed to be exponential and track early events in Italy, which was in a crisis with 14,000 cases, 1000 dead and the country shut down. The US epidemic is at an earlier stage with more than 1629 cases and 41 dead according to the CDC.
It is not enough to count sick people. Many people have subclinical infections and do not progress to symptoms but are still capable of infecting others. Why such people are not seriously affected is a conundrum, or if you are an optimist, a clue. The human population is diverse, and some may have primed immune systems that protect them. An answer will come, and it will be critical to stopping this and perhaps future epidemics. Even if we can cut the production time of vaccines in half, to six months, to depend on vaccines for every outbreak of a new and dangerous virus would result in serious trauma to society. Perhaps there are ways to make a disease less severe in early days, as we will see when we come to the Innate Immune System.
In the meantime, large swaths of the seemingly healthy population, especially in hard hit areas, must be tested for coronavirus to determine how many silent infections there are. Testing distinguishes coronavirus from other common infections and reduces hospital visits. It tells us the state of the epidemic on a local basis. Molecular testing also picks up the effects of hand washing and social distancing, and when drugs or vaccines become available their effects can also be studied without waiting for symptoms.
We have been monumentally bad at delivering test results. It is not for want of scientific skill, but rather regulatory and other hurdles intrinsic to our decentralized medical system and democratic society. Depletion in government agencies that normally prepare for such disasters has also had a serious negative effect. CDC made an error in preparing test kits: one of the reagents was contaminated by fragments of viral genome, which made the test useless. No other tests were approved and the one that was had to be sent to CDC for processing, which was a time-wasting process. WHO had offered a working test, but CDC declined to use the WHO virus test. The CDC is a discovery, diagnostic, and detection focused agency; they are not equipped for large-scale manufacturing, which is an underappreciated skill of its own. Pharmaceutical companies have the facilities and expertise for this task. Mistakes and malfunctions, scientific, administrative, and political, will be sorted out in what I expect to be a damning after action report.
For the very sick, the task is to find hospital beds and medical staff. Physicians and nurses need ventilators and oxygen to keep people breathing when their lungs are severely affected. Fatalities increase when hospitals are overwhelmed, and the supportive measures of modern nursing and medicine cannot be brought to bear. Exhausted nurses, physicians and staff are a recurring theme in this tragedy. Another theme is their heroism. There is not enough medical reserve of personnel in this country to deal with a pandemic such as this one. The Public Health Service has about 3500 nurses and doctors to deploy, and that is not enough.
People can help decrease the rate of infection by avoiding crowds, hand washing, and mask wearing. Avoid surfaces that are coughed on or touched by many people: door handles, gas pumps, faucets, pens, handrails, and elevator buttons where the virus can last for days. These methods cut down the infection rate and avoid breaking the hospital system. It is inconvenient for everyone, but it works.
Masks, Mistakes, and Progress
In March and April people were arguing about masks and disease prevention. Virologists and others initially thought that because the Covid-19 virus is so small, it would pass through a normal surgical mask. We were wrong, fortunately. [I should have been more cautious. A colleague and I used to work with Legionella pneumophila, which causes Legionnaire’s disease.These bacteria have an extraordinary life historyand come packaged in membrane sacs. The concentrated bacteria in a vesicle seem necessary for successful infection.] Viruses also come in lipid droplets, which, from the point of view of the virus, may be adaptive, because droplets (more graphically, think gobs) deliver vast numbers of viruses. One paper I read said that it takes about 1000 virus particles to initiate a SARS-CoV-2 infection, but that seems arbitrary. Masks block droplets, if not single bacteria. The technology of masks can still be improved, although N95 masks are very effective. Improvements that let people work may be possible; there will be future epidemics.
When people inhale droplets of mucus containing SARS-CoV-2 corona viruses, the viruses populate the upper respiratory tract and then sink more deeply into the lungs. If they survive the clearing power and barriers of the airways, the viruses enter human cells and replicate in the throat and lungs and set off a strong innate immune response and then an adaptive, antibody based immune response, both described below.
A review on airborne transmissionby Drs. Kimberly Prather, Chia C. Wang and Robert T. Schooley was published in Science Magazine. They distinguish large infectious particles and small ones—both emerge from a cough or just breathing by asymptomatic individuals. The largest droplets, a tenth of a millimeter in diameter could contain millions of viruses but sink within the now famous, but arbitrary, six feet. These respiratory droplets contaminate surfaces where virus particles may remain infectious. These data are a bit fragmentary, but they are helpful.

A cough and a strobe light. A lot comes out—in two forms. The larger blobs of lipid can contain thousands of viruses. The lighter particles float in the air as aerosols. When inhaled, their many viruses can breach the body’s defenses.
The larger particles are about 1 micrometer in diameter and could contain thousands of viruses, each with a diameter measured in nanometers, or 1/1000th of a micrometer. Aerosol particles are smaller, accumulate in room air and are too light to sink rapidly. Aerosols have a long history in microbiology. Louis Pasteur saw them on beams of light in dark rooms and knew in 1864 they were a source of contamination. (Viruses as we know them were not identified until the 1890’s, although the word existed to connote something morbid and disgusting).
Once a particle containing viruses enters cells in the lung or infect cells in the sinuses and throat, new viruses can be made immediately because the virus contains messenger RNA that can be directly translated into new virus. Think of a thousand virus particles attacking a small area of the lung surface, a form of viral Shock and Awe. Our immune systems, if unprepared, do not have much time to repel the attack. Remember the patient in Wuhan who had hundreds of millions of viruses extracted from a site deep in his lung.
Our defenses before and during an epidemic are largely limited to public health measures until there is a vaccine, drugs, or other preventative treatment. For the scientific paper behind these thoughts, enter: DOI 10.1126/SCIENCE.ABC6197 into any search engine. The information is readable and free to download. It is part of a movement for free access to scientific papers. DOI stands for Digital Object Indicator—not poetic perhaps, but it gets you to the evidence.
The Structure and Necessity of Clinical Trials
All vaccines, drug treatments, and therapies pass through clinical trials, which have rules derived from painful experience that include cheating by investigators, using patients who have not consented or who are not capable of consent, and conflict of interest on the part of the investigators. The idea is to protect volunteers and patients and to do no harm, as well as getting a reliable idea of how effective a vaccine or drug is. Getting permission to start a human clinical trial includes an analysis of the likelihood of success, the pre-trial evidence, and statistical requirements.
Clinical trials must register with an organization at the National Library of Medicine at the NIH. Anyone can find the list of clinical trials for a disease or condition at www.ClinicalTrials.gov. If you are interested in joining a SARS-Cov-2 clinical trial, you will also find medical centers involved in the trials at that website (enter code identifier NCT04470427 for the Moderna vaccine). Clinicaltrials.gov lists clinical trials for all diseases, drugs, devices, and conditions, with contact information. There are many thousands, but there is a good search function.
Trials have three stages. The first, which uses only a few volunteers, makes sure a vaccine or drug is safe and establishes dose. The second phase engages more volunteers and looks for adverse reactions and the activation of the immune system (in the case of vaccines), but does not yet concentrate on efficacy, although with hundreds of people, positive or negative effects may be found. The third phase tests protection from infection and requires thousands of volunteers of all ages and ethnicities to get statistically significant answers. The number is usually 30,000 in the current vaccine tests. Neither physicians nor patients know whether an injection contains vaccine or placebo; hence the trials are called double-blind. Well-designed clinical trials are the most critical part of solving Covid-19 or any other problem. They are expensive and may fail—the FDA keeps a humbling list on its website of vaccines that looked to be excellent through phases I and II, but which failed the final test in Phase III. The Merck vaccine was one.
After a late start, the British are making progress. They have the advantage of the National Health Service and its hundreds of hospitals (one of which saved the Prime Minister, who seems suitably grateful). The NHS uses one clinical trial protocol and a unified reporting system for several hundred hospitals that are equipped and trained to do this work. The British system is called RECOVERY (Randomized Evaluation of COVID-19 Therapy) and has already yielded results.
The NHS proved the value of dexamethasone, an anti-inflammatory steroid drug used for Covid-19 patients to control inflammation. Other data showed that hydroxychloroquine is useless. Several drugs that are useful against HIV and might have worked against SARS-CoV-2, even should have worked, did not help. According to Science Magazine reporter Kai Kupferschmidt, these three results changed the standard treatment of Covid-19 over a few weeks.
Interferons, a group of small proteins that are produced by defensive cells in response to infection are critical for inflammation and are induced during the innate immune response (see below); they may be useful in slowing a Covdi-19 infection. This and other leads are now being tested in various formulations.
Monoclonal antibodies that bind to the SARS-CoV-2 virus and neutralize it are being tested, for treatment and prevention. Former President Trump believed they cured him and perhaps they did, but proof awaits clinical trial completion.. A January 2021 study from Regeneron, a pharmaceutical company, concluded that they are useful early in the infection during the period before a patient makes his or her own antibodies, but not after.
One of the stranger stories I came across in reading this literature involved antibodies from llamas, alpacas, and camels. Antibodies from these species are one tenth the size of human antibodies and it is possible to isolate monoclonal versions that bind to the Spike protein of coronavirus with extraordinary tenacity, which means that the antibody might block virus entry into lung or other cells. Camel and alpaca antibodies are stable, and the authors of the paper are testing them by inhalation against SARS-CoV-2 in animal experiments.
Why are mRNA Vaccines Different from Earlier Vaccines?
Some people, confronted with the SARS-CoV-2, decline the vaccine because they think it was developed in a rush. The question is not whether the Moderna or Pfizer vaccines were developed in a rush, but rather why traditional vaccines took so long to make. The classical vaccines were made in the days before nucleic acids could be sequenced and before all viruses could be compared at a detailed molecular level.
Polio, measles, or mumps vaccines were created in the 1950’s and 1960’s. They are all RNA viruses like SARS-CoV-2. At the time, there was no way to determine their RNA sequence because sequencing methods were not developed until the late 1970s. These and other vaccines were triumphs, but we did not know the genes that coded for essential functions of the virus or how they were changed in a vaccine. We could see the shape of viruses in electron microscope images (viruses are too small to see in light microscopes), but vaccines took a long time to make. To make a vaccine, scientists had to inactivate or weaken the virus. Inactivation with formaldehyde killed the virus and sometimes this works as a vaccine, but often live viruses provoke the immune system better than a virus or bacteria killed with formaldehyde. Thus, the Salk polio vaccine contained dead virus and the Sabin vaccine contained a weakened but live virus.
The classical vaccines were created by growing the viruses in cells in petri dishes, harvesting the virus and then growing them again. And again. This is called passaging the virus and eventually a virus emerges that can activate an immune system without sickening the host. Finding a mutant virus that could activate the immune system without causing disease often took years.
The first anti-viral vaccine, against rabies (also an RNA virus) was a daring venture into the unknown, depending on your point of view. Desperation played a role and fortunately, the vaccine worked, if given soon enough after an animal bite. Louis Pasteur and his colleagues did not even know about viruses, but they were effective scientists and knew how to use controls. They were also a little lucky.
SARS-CoV-2 vaccine candidates based on sequencing information entered phase 1 clinical trials in the late spring and early summer of 2020. They are tested for safety, dose, and the immune responses they provoke. The vaccines usually present humans with the coronavirus Spike protein whose role is to bind to a protein that protrudes from human cells. Starting in February 2020, it will take at least a year to complete Phases 2 and 3. There will be intense pressure to speed the process up. That involves risks, but they may be inevitable. (The timing turned out to be right and we perhaps did better than expected.)
At the end of July 2020, the data from phase 1 and 2 trials of three vaccine candidates were released—one by Moderna, one by Pfizer, and the other by the Oxford/AstraZeneca vaccine team. All vaccines provoked a robust immune response in healthy volunteers.
The vaccines employ different strategies to provoke the human immune response, and both have begun large phase 3 trials to determine efficacy. Two have been approved for emergency use in the US, the UK, and other countries. The Oxford/AstraZeneca vaccine is being administered in the UK and other countries and is available in the United States (it ran into difficulties but will still be useful). The Johnson and Johnson vaccine began distribution began on March 2, 2021. There are many other candidates, but the important part of this process is not only the creation of the vaccine, but the size and excellence of the clinical trials, including the power of their statistical analysis.
Waiting is the Hardest Part
On October 25, 2020, we were waiting for medical centers that administered Moderna vaccine or placebo in Atlanta, Houston or other hotspots of infection to report on the numbers of people in their trials had Covid-19. From my inquiries at the NIH, the Moderna vaccine is the only one in a Phase 3 trial that is fully subscribed—30,000 people have volunteered and have had both shots. The similar Pfizer vaccine was a little behind. Other vaccines, from Oxford/AstraZeneca, Novovax, and Johnson and Johnson were all around the same stage in October 2020.
Before starting their Phase III trial, Moderna and all other companies, with the cooperation of FDA scientists and statisticians, had decided on how many patients with Covid-19 had to be reported before the books on the double-blind trial were opened. At that moment investigators learned which patients had received the placebo and which the Spike coding mRNA. It is a surprisingly small number: 94 among 30,000 volunteers. As the trial went on, the number of people with symptoms increased to the hundreds.
In the best of worlds, the sick patients would have received placebo and all the people who received vaccine would be healthy. If both groups had an equal burden of disease, the vaccine fails. If the people who got vaccine had half the cases of the people who got placebo, that is defined as a success. For those involved with the development of these vaccines, this is a nerve-wracking moment.
We need to know a lot about each sick patient in the trial. How sick were they? How long did they remain sick? Did they need oxygen or a ventilator? Did anyone die? What was the viral load in each patient? Is the patient making antibodies and T-cells? Are there side effects? Do tests in Atlanta, Toronto and Phoenix report comparable results? A lot of information can be teased out of a well-designed trial, including fraud in reporting. I include the summary of the Pfizer vaccine data from the New England Journal of Medicine. The DOI number pasted into your search engine will take you to the full paper.
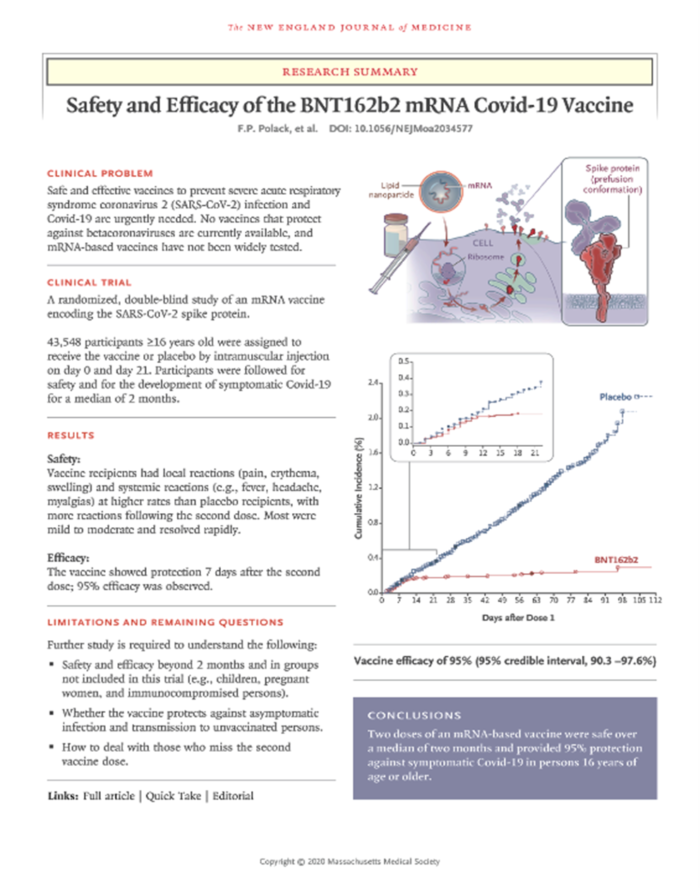
The original data from Pfizer: The graph on the lower right tells the story. The red line represents the number of vaccinated people who got sick over time. The blue line represents the number of sick people in the unvaccinated population.
Almost all the volunteers who received active vaccine avoided Covid-19, or at least getting seriously ill, which is what counts. Moderna and Pfizer prepared applications for Emergency Use Authorizations. A famous standing committee of the FDA with independent reviewers studied the data and in mid-December they voted and gave preliminary authorizations. In February 2021, all had received an Emergency Use Authorization by the FDA. In June 2021, the Novovax vaccine was approved. It is a novel vaccine and is important because it has no RNA or DNA sequences in it. By law, a vaccine cannot be licensed until it has been used for a certain time and shown to have few and rare side effects.
……..
The anxiety of waiting for vaccine results has a history that is worth a short digression. Louis Pasteur and his colleagues made the first designed vaccine in 1879, for anthrax infection of sheep. There was a clinical trial at a farm in a town call Pouilly-le-Fort, a village to the southeast of Paris. Dr. Patrice Debré, a biographer of Pasteur and himself an immunologist, called it The Wager of Pouilly-le-Fort. Twenty-five sheep were vaccinated and 25 were not. After two weeks, the vaccinated sheep got a boost of vaccine (a lot like the Moderna and Pfizer coronavirus vaccine schedule). Two weeks after that, all fifty sheep were given a lethal dose of Bacillus anthracis. (Prof. Debré’s biography of Louis Pasteur has an extensive account of the anthrax experiments and has been translated into English).
Louis Pasteur came to Pouilly-le-Fort to inoculate the sheep with the help of Emile Roux and Charles Chamberland two of his most effective assistants, but Pasteur did not come to see the result. Overcome with anxiety, he paced in his laboratory, while assistants carried out the injections of lethal anthrax and then went back to Paris. According to Professor Debré’s account, a large crowd gathered for the outcome. It included reporters, scientists and heads of agricultural societies as well as many farmers. People knew the importance of the outcome and Louis Pasteur was famous for solving other problems, so there was high hope. Success would change medicine and agriculture; failure would set back vaccines and medicine for decades. Two days after injecting virulent anthrax, nobody from the team was at the farm in Pouilly-le-Fort, and it was left to a veterinarian, Dr. Rossignol, who had organized the event, to send a telegram to Pasteur. “Succès épatante! (Stunning success), it said. Twenty-five sheep were dead or dying; 25 vaccinated sheep were healthy.
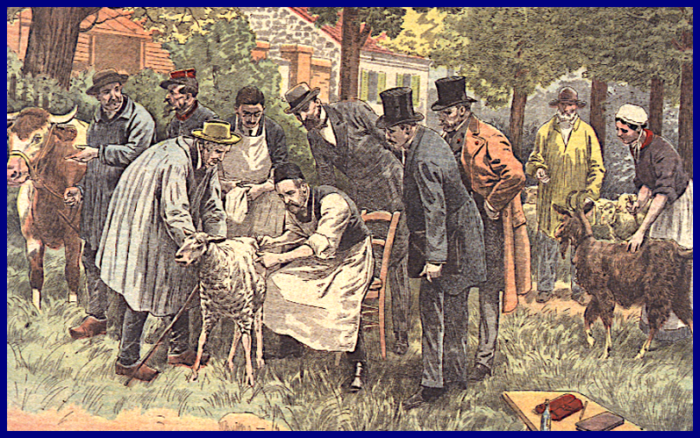
The First Vaccine: Sheep inoculated with weakened anthrax at the first vaccine trial since smallpox in 1799. Pouilly-le-Fort, France, 1881. This is one of several artists’ renditions. Notice the cow on the left. Some cows were added as a gesture to local farmers.
Pasteur and his colleagues went on to make vaccines again chicken cholera and rabies. The rabies vaccine was the first used on humans and convinced the public that vaccines and science could transform medicine. I cannot tell that riveting story here, but there is a film, The Life of Louis Pasteur, starring Paul Muni, which does tell his story. The film won an Academy Award for best picture in 1936 and is available on Netflix. It compresses the story, but it is not bad for a Hollywood rendition.
……..
The placebo arm of the Pfizer, Moderna, or any other trial becomes ethically untenable when the results are clear. Placebo treated patients must be notified and offered real vaccine. A friend who was in this trial wasnotified that she had received placebo and got the vaccine in early January 2021. All treated patients, in the case of the Moderna and Pfizer vaccines (and others), will be followed for two years to learn how long antibody and T cell responses last and to look for late side effects. Those results, properly studied, could lead to licensing of the vaccine. In the meantime, vaccines are administered under an emergency use authorization, an EUA.
In January 2021 there was enough FDA approved vaccine to distribute widely. Some vaccines need to be frozen on dry ice, but Fed-Ex, UPS and other companies are prepared for frozen packages. Scientists and clinical labs know how to handle cold temperature—many of their supplies arrive on dry ice. Cold temperatures will not be the rate-limiting step. The Johnson and Johnson vaccines should be ready shortly (they are), as will the novel Novavax vaccine, which has been approved. Distribution will be through established channels. I do not believe the military will be heavily used, except to vaccinate their own people and dependents (no small task) and perhaps for distribution in difficult places. [National Guard and Regular Army troops have effectively assisted the vaccine rollout. They provided order, calm, and confidence, as a physician friend who got her vaccine at the Javits Center in New York City recently told me. Kudos to them.]
The worries about distribution and injection are real, but we have done this before. In 1947 a smallpox outbreak hit New York City. Within a week, millions of people had been vaccinated, an effort that remains a seminal moment in the history of public health. SARS-Cov-2 vaccination will also be such a moment. In the end, it is a question of character. Great nations have resilient institutions.

In 1947, New York City had 12 cases of smallpox and two deaths. Six million people were vaccinated in a little more than a week.
The Adaptive Immune System Makes Specific Antibodies and T-cells
But not for a while…
The first scientists to make a vaccine were led by Louis Pasteur, as we have seen. Pasteur had made the discovery years before that fermentation is carried out by minute organisms, including bacteria and yeast, and without them wine cannot be made, bread does not rise, infections do not occur, and meat does not rot. There was extraordinary, even furious objection to the germ theory of fermentation and disease, but Pasteur was right. Emile Duclaux, one of his students and later head of the Pasteur Institute, called the germ theory fertile: it gave rise to microbiology and immunology, vaccines, surgery without infection, and safer childbirth. It eliminated infections in milk and water, led to better wine, beer and cheese, and created industrial advances too numerous to list. Over time, child mortality from infectious diseases plummeted. Even now, fertile is not a word normally associated with a theory, but it should be. Fertile theories make people think.
The smallpox vaccine, in 1799, was first, but it was an outlier, preceding others by about 80 years. Why the delay? It took that long to create the idea and substance of microbiology and the germ theory of disease. Once Louis Pasteur, Robert Koch, Joseph Lister and others knew that a bacterium caused anthrax, Pasteur and his students managed to weaken Bacillus anthracis and that gave rise to the first vaccine against animal diseases, after smallpox. The opposition continued to be furious, but Pasteur knew the power of his ideas and he was a formidable debater and showman. He reminded his opponents that Fortune Favors the Prepared Mind. They retreated, but it took decades.
Fortune also favors the prepared immune system, about which Pasteur knew nothing, except that animals and people became resistant to reinfection. He knew that when people recover from an infection, they are usually immune and can then safely nurse the sick. He knew about cowpox and the immunity it provided to smallpox. Deciding to make a fresh vaccine was a leap.
…….
Let’s leap forward about 140 years and ask how proteins are made and how immunity occurs. Most of the Covid-19 vaccines are designed to present a SARS-CoV-2 Spike protein to the human immune system and provoke the production of circulating antibodies that bind to the virus or killer T-cells that recognize infected cells and punch holes in them.
The Spike protein is a long string of amino acids, small molecules that snap together like Lego pieces. The acid group of one reacts with an amino group of the next to establish a short chain that grows by addition. The first amino acid in a chain is always methionine; the second could be any of nineteen others. By the time we get to three amino acids there are 400 possibilities, then 8000 (400×20) and so on. Each of the twenty amino acids has different groups of atoms that create shapes, surfaces, helices, and other structures in a finished protein. The number of possible protein shapes is astronomical, just as it is for a phonetic alphabet can code for all of literature.
The Spike protein, on which our vaccines depend, has 1200 amino acids that fold up to form a spike shape. Evolution took advantage of random mutation and the diversity it provides to select an order of amino acids that folds into a Spike shape. Spike proteins also binds tenaciously to ACE2, a protein that is part of a system that regulates blood pressure in lung cells and blood vessels. The vessels are attacked by SARS-CoV-2, causing clots, strokes, and heart attacks.
The Spike protein is still changing—genetic changes occur spontaneously and can lead to a Spike protein that binds ACE more tightly, making the virus more potent. That is what appears to be happening with the UK, Brazilian, and South African and Indian (delta) variants of the coronavirus. It is classic evolutionary biology—huge diversity in a large population, followed by natural selection of variants that lead to more production of a single mutant virus with better ability to replicate.
After a vaccination or illness, the immune response subsides, but antibody-producing B cells and cell-killing T cells are banked to provide a memory of past infections. When infection with a previously encountered virus occurs, thousands or millions of banked lymphocytes start to divide and quickly produce enough antibody or killer T-cells to block or minimize the infection. These are called memory B and T cells. With some vaccines the banked cells last a long time (measles, yellow fever, smallpox, mumps and rubella), but with others, a few months or years (whooping cough, tetanus, influenza). Your cache of memory B cell and T cell is the most important bank account you own.
A normal human being facing a novel virus is at a disadvantage because it takes time, perhaps two weeks to produce to produce antibodies and killer T cells by expansion from single cells. Most B-lymphocytes in the blood, spleen, or bone marrow are dormant, their genes are shut down, their DNA folded up. Under the microscope, the lymphocytes form a sea of blue cells. Each lymphocyte has a different antibody protruding from its surface. One lymphocyte cell; one antibody; billions of different lymphocytes each waiting for something to bind.
Suppose we inject the owner of these dormant B-lymphocytes and T-cells with Spike protein. What happens? Among the billions of lymphocytes, a few recognize the shape of a Spike protein fragment. The fragments have been predigested, (that process is understood but beyond us for the moment). A signal goes to the dormant nucleus, notifying the cell to unwrap its DNA and start to divide. In two weeks, there will, in theory, be 213 B or T cells (more then 16,000) with the same antibody on their surfaces. We call this clonal expansion of a single cell.
There is a lot else going on. We cannot have antibodies or T cells reacting to our own proteins or we will get autoimmune diseases. Such self-reactive lineages are killed off. B cells that react with the host are killed in the bone marrow, reactive T cells are killed the thymus. As the cells mature, rearrangements of DNA take place and regions of the gene are mutated to create even greater antibody diversity. As B-cells mature, their antibodies, thousands per minute per cell are released into the blood stream. A second booster shot, as in the case of anthrax or the current coronavirus, increases the number of cells and antibodies.

Clonal Expansion creates a lot of antibody-producing cells, but not immediately. Imagine a cell that is programed to produce antibodies (1): the lymphocytes, whether B or T cells derive from stem cells that live in the bone marrow. They have an immunoglobulin facing outward on the cell membrane. Each immunoglobulin is different. In the diagram there are three variants caused by mutation. Such mutations do not happen anywhere else in biology. Any that react with the host’s proteins die. The rest leave the bone marrow (or thymus in the case of T cells) and enter the circulation to lymph nodes. Here they might meet and stick to a Spike protein. If they do, they start to divide and expand their clone.
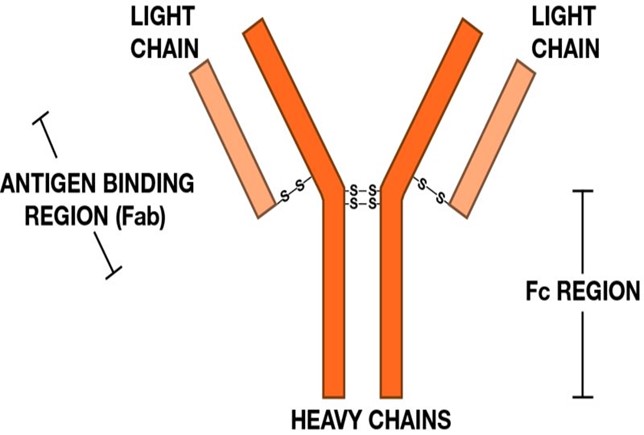
The shape of an antibody: Normally antibodies have two chains that are stuck together. Only a few parts are mutated in what is called a variable region (Fab in the diagram). An antibody can be produced that will bind to almost any chemical, but usually to a fragment of protein. The non-variant fragments (Fc) attach to cells that ingest antibody bound to a virus. The virus gets eaten.
It takes a lot to become a decent B cell or T cell. Most of all it takes huge numbers and a great diversity of antibody. B and T-cells do not divide without help from other cells that secrete proteins that stimulate them to divide, but two weeks after injection of Spike protein, we have lots of antibody and many T-cells. If the infection has not already caused terrible damage, it will be controlled.
The Innate Immune System Defends Us Early in an Infection
In the middle of an explanation of clonal expansion of lymphocytes, a woman in the second row of my class, once asked as politely as she could, “Dr. Kessin, are you sure? This sounds like baloney to me.” She had a point, after all. I explain that immunology is confounding because it breaks so many conventions of biology, that it is hard to believe. How is it possible that the immune system can recognize millions of chemicals, and not recognize a person’s own molecules? How does it avoid creating autoimmune diseases? How does it sequester memory cells? How does it deal with novel infections if it takes two weeks to turn on the adaptive immune system?
The idea that tiny bacteria can kill was also hard to believe in the 19th century. It was easier to believe that the little rods found in the blood of anthrax infected animals were the product of a disease rather than its cause. Immunity as a problem has taken more than two hundred and twenty-five years to work out, starting with Jenner’s smallpox vaccine. We are not done.
The messenger RNA used in the first two vaccines was once an abstract concept, something that had to exist but could not be seen, like dark matter. That was in the early 1960’s. That problem was solved and now we have mRNA vaccines. It’s not a miracle, just hard work, imagination, time, and the money for basic science and development in universities, national laboratories, companies, and elsewhere around the world.
Allow me another short digression: From where did the support for basic science research come? In late 1944, President Roosevelt and his science advisor, Vannevar Bush, were thinking about the US government’s vast wartime investment in basic science when Roosevelt asked what would happen to basic science when the war ended? Bush said it would collapse. Roosevelt decided that should not happen. Vannevar Bush was an engineer from MIT whose book about basic science inquiry, The Endless Frontier, became a seminal text in the history of American science and industry.
There was an enormous reorganization of government agencies after the Second World War, including of the military, intelligence agencies, diplomacy, and more. Sometimes lost in the bigger picture is the reorganization of science—The National Science Foundation was founded to do basic research, and the NIH was re-organized. National Laboratories were founded (we have 20, run by the Department of Energy). A large amount of money was dispersed to universities, much of it for basic research, which has no immediate practical or commercial application. The NIH and NSF do not hand out sacks of the money. The application process is rigorous, not to say terrifying.
If a scientist had an idea—say, to find out what Toll Receptor proteins do in fruit flies (no kidding), there was a way to apply to the NIH for money to test that idea and to train young scientists and undergraduates in the process Some of the titles of these projects could drive non-scientists like Senator William Proxmire mad, but the totality of ideas and people working in labs, gave rise to advances in biology and medicine and the physical sciences in the United States. One of these was the innate immune system, which 35 years ago was largely unknown.
The innate immune system is a collection of mechanisms that protects cells and animals against viruses, bacteria, fungi, amoebae and worms; it is present in all nucleated cells. The adaptive immune system, with its antibody repertoire, is confined to vertebrates. (Bacteria have other mechanisms of defense, including CRISPR, which, while we have been distracted by Covid-19, has been harnessed to treat sickle cell anemia, thalassemia, and other inherited diseases.)
The innate immune does not make antibodies or T cells and retains no memory of past infections. It limits threats and alerts the adaptive immune system about the kind of pathogens that are probing our airways or blood stream. Innate immunity is always present; it is complex and a bit of a blunt instrument, but fascinating. Without it we are at risk because a person who has never seen SARS-CoV-2 will not have memory B or T cells for two weeks. In 1997 Charles Janeway and Ruslan Medvedev at Yale showed that a Toll like receptor could induce an adaptive immune response in animals. Toll-like receptors have distinctive properties and soon there were a dozen.
Imagine a lipid droplet containing thousands of virus particles inhaled by a victim. Those viruses will escape onto the membranes of cells in the nose, throat, or lung. The virus binds to the protein ACE2, the landing site for the Spike protein and gets pulled into the cell, where it unwraps, and starts to make proteins from its RNA. This sounds ominous and it may turn out that way, but macrophages in the throat and lungs system have also sensed the invaders. Normally they eat invaders, but they also let the body know what is attacking it, whether an RNA virus, a bacterium, tuberculosis, a worm, or a fungus.
The innate immune system’s antennae are 10 or 12 proteins called Toll-like receptors. They face out of immune cells and sample the environment for invaders. Finding an RNA virus like SARS-CoV-2 sets the innate immune system off (the innate immune system hates viral mRNA, to be anthropocentric for a moment). The Toll-like receptor TLR7 recognizes general properties of an RNA virus and signals the cell to turn on the genes involved in viral defenses. They attract defensive cells to the lung or other site of infection. This police force includes neutrophils that arrive in the lung’s capillaries, penetrate the capillary wall, and get into the virus infected air sacs. They accumulate quickly and die in the cause of suppressing the infection. Their remains are one of the main constituents of pus.
If there is too much activation, a so-called cytokine storm can occur and that is destructive because many of these recruited cells carry digestive enzymes that are released, causing tissue destruction and inflammation. As part of inflammation, the lung’s capillaries leak, and the air sacs fill with fluid and defensive immune cells. The process can leave a mess that one of our medical students once described as the remains of a barroom brawl. Inflammation is the event that the dexamethasone treatment and other interventions controls.
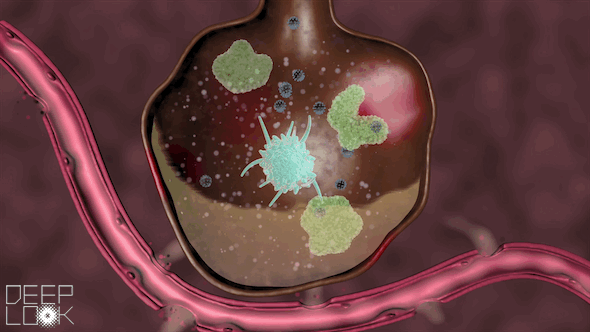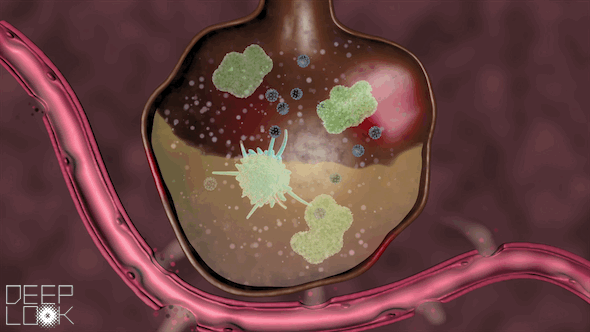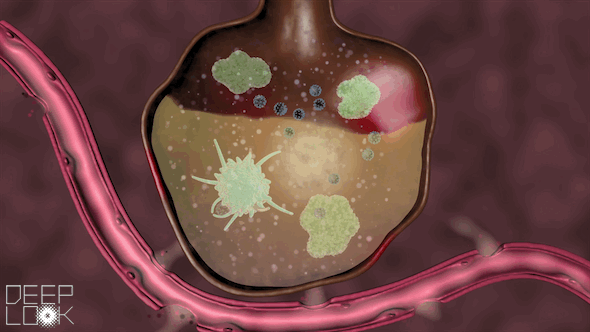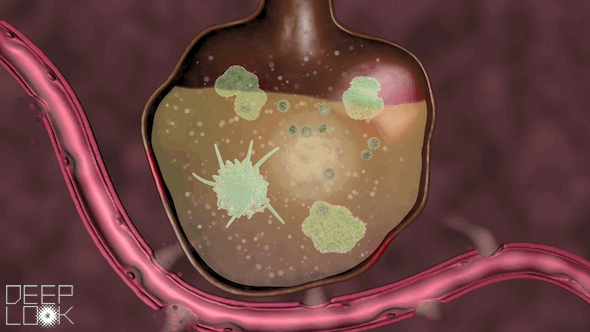
The danger of inflammation in the lungs. Our air sacs are patrolled by vacuum cleaners called macrophages that eat small bacteria and virus fragments and digest them. Macrophages have Toll-like receptors of the innate immune system. When they sense virus RNA, they set of an alarm and recruit millions of neutrophils, another form of digestive cell, which migrate out of the circulation an into the alveoli. Uncontrolled inflammation causes alveoli to fill up with cells and fluid, making it hard to breath. This video was prepared by the science reporters at KQED, the Public Television Station in San Francisco.
If the innate immune system functions well in the week or two after SARS-CoV-2 infection, before the more specific B and T-cells have entered the fray in large numbers, it tends to limit early SARS and other infections. If so, we might ask whether the DNA of very sick Covid-19 patients contains disabling mutations in genes of the innate immune system. At least for some patients, that seems to be the case. Other patients have antibodies against their own interferon, a critical component of innate immunity and they also are more vulnerable. Asthma patients, who have lung inflammation much of the time, seem to do better with Covid-19. Another prediction is that RNA, presented by a harmless virus should inhibit SARS-Cov-2 or other dangerous viruses by inducing the innate immune system? Perhaps the SARS cold virus? I hope so.
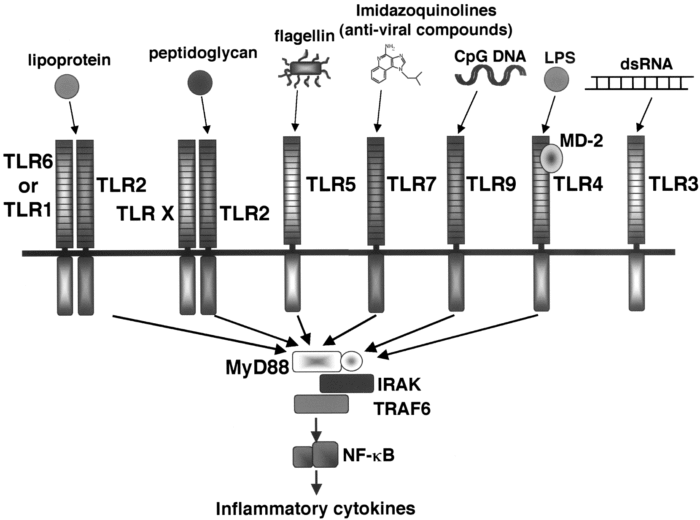
Sensors of Innate Immunity: Toll-like receptor proteins cross the membranes of immune cells to sense what invaders are outside. The perpendicular lines show the Toll like receptor proteins, or TLR’s, which have strange repeated sequences of amino acids. TLRs bind and detect the RNA or DNA of viruses, and other TLR molecules on the surface detect bacteria, fungi, or worms. The proteins inside the cell, MyD88 and others, indicate how the information detected by the TLR induces defensive genes. When they detect a virus, the TLR3 and TLR7 Toll-like receptors activate cells to produce interferon or cytokines that in turn induce other genes that slow the infection.
Nature often beats us to interesting treatments. Perhaps, giving people harmless RNA will stimulate the innate immune system enough to provide protection. We need some general defense to cover the first days of new virus infections. Vaccines are superb instruments of medicine, but it still takes a year to make and test a vaccine. In a year of SARS-CoV-2 the United States lost 600,000 people.
Who Will Deal a Pandemic in 2040?
Nothing is more valuable in the world of infectious disease than the human ability to watch for calamity coming over the horizon. That is what the CDC, the World Health Organization, various well-funded private agencies, and the specialists in the Department of Agriculture do. No organization or country is big enough to watch for all impending diseases and to do initial studies when they find one.
At this moment in the Covid-19 Odyssey, President Trump and his administration are lost on a wine-dark sea (November 2020). They have been infected but they do not know what to do or what the course of the disease will be—only that it is out of control. The administration has essentially given up without turning the matter over to the incoming Biden Administration. The Centers for Disease Control and Prevention have not been consulted in some time. CDC has taken a battering, but they know there are lives to be saved by simple public health measures before the vaccines arrive. They will rally and the new CDC Director, Dr. Rochelle Wilensky, has an excellent reputation and is free to do what she thinks best.
My question here is what will happen when we face the next pandemic? The scientists and physicians who will confront a pandemic in 2040 are now in high schools around the world. Investment in people is what we did during and after World War II and after Sputnik—and that surely worked. There is nothing better to engage students in science and medicine than a good story. I was attracted to science by a series of elegant medical detective stories by Berton Roueché, as were many other scientists and physicians. His bell-clear stories appeared in The New Yorker beginning in the 1940’s and continued for decades.
The first Roueché story I read was Eleven Blue Men. It begins: At about eight o’clock on Monday morning, September 25, 1944, a ragged aimless old man of eighty-two collapsed on the sidewalk on Dey Street, near the Hudson Terminal. A little further on we learn that: The old man’s nose, lips, ears, and fingers were sky-blue. Sky-blue? Really? Not just a little blue, as in cold, but sky-blue? I needed to know what happened to this old guy and ten men like him.
Soon we meet Dr. Morris Greenberg and Dr. Ottavio Pellitteri of The New York Department of Public Health—the chief epidemiologist and a field epidemiologist. Dr. Pellitteri traced the blue men to bleak Bowery hotels and then to a restaurant where they all ate. He discovered that all these men had eaten oatmeal with sodium nitrite (used to cure meat) rather than sodium chloride (table salt). Ten of them recovered and the blue color gradually faded. One succumbed to tuberculosis, then common. Berton Roueché’s stories about health detectives from the New York Health Department and the CDC are extraordinary—there is a fine anthology called The Medical Detectives. If you are considering nursing, science, medicine, or journalism, or if you just like clear writing, read his stories.
Decades after I read about Eleven Blue Men, I was teaching medical and PhD students at Columbia University’s Irving Medical Center when a physician-investigator from the New York Department of Public Health called me. She needed a reference for a young researcher from my lab; he had applied for a job as a disease detective on her service. We talked for a while (he got the job) and then I asked if it was true that the Health Department conference room was named for Berton Roueché. She seemed delighted that I had asked. It is! He’s my hero. He changed my life! We talked for a pleasant half hour.It’s good to have kindred spirits.
Mr. Roueché died in 1983, but CDC and the New York Health Department continued to solve cases. For example: In April 1993, a young Navaho woman on her way to a friend’s wedding in Gallup, New Mexico developed trouble breathing. Physicians and nurses at the Gallup Indian Medical Center tried to help but she developed a devastating pneumonia and died. A week later so did her fiancé. Twenty-six people, Native American, Hispanic and Caucasian, most young and healthy, fell ill, and thirteen died. The New Mexico Department of Public Health called the CDC.
The lungs of these patients were white with liquid that blocked X-rays. There was one clue: X-ray images looked like those from a Hantavirus patients seen years before in Korea. Hantavirus infections had never been found in the United States, but the CDC scientists knew that mice carried it and reasoned those mice around the victims’ house might carry the virus. The ground under and around the house was permeated with mouse urine and feces, both with high levels of Hantavirus. When the ground dried, the wind whipped these leavings into an aerosol that the victims had probably inhaled. Hantavirus is not confined to the Southwest—it is ubiquitous. A young man on Long Island got sick with Hantavirus pneumonia after sweeping his garage in the spring.
I remember a seminar at Columbia University Medical Center in 1994 at which the CDC investigator explained how his team had solved the case. The lecture hall seats about 150 people, and it was packed with scientists and physicians, many standing in the aisles, on the side, and the spaces at the back. When the speaker showed X-ray images of the victims’ lungs, there was a gasp; one did not have to be a physician to know that these people could not breathe, and that Hantavirus was the cause. The case was solved in days by removing the mice and decontaminating the ground..
The novel Hantavirus was named Sin Nombre (no name). Sin Nombre virus is a nasty piece of work, one of our most worrisome, for which there is still no vaccine or treatment. Navaho elders had made the association between mice and pneumonia long before. CDC issues guidelines for diagnosis and provides training for physicians practicing in the Four Corners Region of the Southwest. Public health measures keep Sin Nombre at bay. Unlike SARS-CoV-2, Sin Nombre is not transmitted person to person. Imagine if it had been
Sources and Other Light Reading
The Plague Year by Lawrence Wright, The New Yorker, Jan. 5, 2021
Rage by Bob Woodward (unique sources, as usual)
CDC: https://www.coronavirus.gov
NIH: https://www.nih.gov/coronavirus
NCBI SARS-CoV-2 literature, sequence, and clinical content: https://www.ncbi.nlm.nih.gov/sars-cov-2/
This Week in Virology, TWIV. A podcast by Professor Vincent Racaniello and colleagues. It covers all of virology in detail.
The Famine of Men, 2014, a novel about a young virologist who discovers a virus that kills the cells that make testosterone. The book is about how science works and the people who do it. From AuthorHouse or Amazon. By the author
Relevant Columns from The Body Scientific in The Lakeville Journal: See Richardkessin.com for columns on other subjects.
Part 1 of 2 In 2014 and 2015, Ebola virus infection killed 8,698 people in Sierra Leone, 3,337 in Guinea and 3,955 in Liberia. There were survivors…
03/14/2016
09/13/2017
Ebola, Ça suffit! (Ebola, that’s enough!): Progress has been made on Ebola and other diseases
Part 2 of 2 Ebola virus periodically leaps out of a reservoir in Africa and causes high mortality in humans and great apes. We do not know for sure…
The Ebola threat: What we learned from it
We learned that some people panic, and others do not. We learned that physicians, nurses, hospitals, the CDC and the NIH make mistakes, but we have…
11/07/20
09/06/2017
How a rabies vaccine captured the public’s imagination in 1885
02/17/2016
Infection and its control: antibiotics
In the last third of the 19th century, the Germ Theory of Disease was a liberating idea that helped extract medicine from near futility.
04/24/2015
When conviction trumps evidence: Lessons of a measles outbreak
Before 1963, almost all children got measles in the United States. Decades later some remember it as a rite of passage, unpleasant certainly, but not a…
04/16/2015
Elie Metchnikoff, World War I and the End of Optimism
We observe the end of World War I on Nov. 11, Armistice Day. For Americans, World War II is the more remembered event, but the calamity of World War I…
10/17/2014
What story does the DNA tell us?
When I was a Ph.D. student in the late 1960s, a scientist determined the exact DNA sequence that encoded the plan for part of a protein. He and his team…
01/30/2012
08/16/2011
In 1735, a diphtheria epidemic swept through Connecticut and the rest of New England. It started in Princeton, N.J., and raged up the coast for several…